Virtual Lab: Titration
Introduction
In chemistry laboratory, it is sometimes necessary to experimentally determine the concentration of an unknown acid or base solution. A procedure for making this kind of determination is called an acid-base titration. In this laboratory process, a solution of known concentration, called the standard solution, is carefully added to a solution of unknown concentration until the mixture becomes neutral. The neutral point of the solution is recognized by an indicator’s color change. If the unknown solution is acidic, then the standard solution will be basic. The opposite would be true if the unknown solution was basic.
We know that the mixing of equal amounts of acid and base ions will create neutral water. At the molecular level, this reaction can be illustrated with the following equation.
H+ + OH- --> H2O
(acid) (base)
This equation states that one mole of hydrogen ions (acid) will neutralize one mole of hydroxide ions (base). Since we can exactly measure the moles of the standard solution, we can assume that the moles of the solution of unknown concentration will be the same at the neutral point. This is called the end-point of the titration. Using the equation MaVa = MbVb, we can use the experimental data from the titration to find the unknown concentration.
Objective:
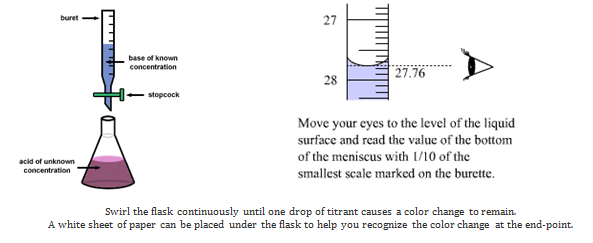
In this experiment the unknown solution will be HCl(aq) and the standard solution will be the base sodium hydroxide. You will know the concentration of the base and the volume of the acid and base used. With this information you can use the titration formula to calculate the concentration of the acid. The diagram below shows the set up.
In chemistry laboratory, it is sometimes necessary to experimentally determine the concentration of an unknown acid or base solution. A procedure for making this kind of determination is called an acid-base titration. In this laboratory process, a solution of known concentration, called the standard solution, is carefully added to a solution of unknown concentration until the mixture becomes neutral. The neutral point of the solution is recognized by an indicator’s color change. If the unknown solution is acidic, then the standard solution will be basic. The opposite would be true if the unknown solution was basic.
We know that the mixing of equal amounts of acid and base ions will create neutral water. At the molecular level, this reaction can be illustrated with the following equation.
H+ + OH- --> H2O
(acid) (base)
This equation states that one mole of hydrogen ions (acid) will neutralize one mole of hydroxide ions (base). Since we can exactly measure the moles of the standard solution, we can assume that the moles of the solution of unknown concentration will be the same at the neutral point. This is called the end-point of the titration. Using the equation MaVa = MbVb, we can use the experimental data from the titration to find the unknown concentration.
Objective:
In this experiment the unknown solution will be HCl(aq) and the standard solution will be the base sodium hydroxide. You will know the concentration of the base and the volume of the acid and base used. With this information you can use the titration formula to calculate the concentration of the acid. The diagram below shows the set up.
Pre Lab:
Define the following words:
Titration- ___________________________________________________________
Endpoint- ___________________________________________________________
Neutralization- _______________________________________________________
Write the neutralization reaction for HCl reacting with NaOH.
What is the pH of the solution at the end point of the titration?
Procedure:
Define the following words:
Titration- ___________________________________________________________
Endpoint- ___________________________________________________________
Neutralization- _______________________________________________________
Write the neutralization reaction for HCl reacting with NaOH.
What is the pH of the solution at the end point of the titration?
Procedure:
- The flask is filled with 10 mL of unknown concentration of HCl. (Click here) Record the volume of acid on your data form
- Phenolpthalein Indicator is added to the flask. (click here)
- Record on your data form the initial volume of base in the buret (click here)
- You will now start adding base (0.25M NaOH) from the buret into the flask to neutralize the acid. The flask is gently swirled as the base is added. A pink color should appear as the base is added, but it will disappear as the flask is swirled. The end-point of the titration will be reached when 1 drop of base makes the solution turn and stay pink. (click here).
- Record the final volume of base from the buret that was needed to neutralize the acid. (click here)
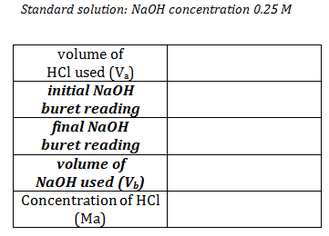
Data:
Calculation:
Determine the Molarity (concentration) of HCl using the data you collected and the titration formula. (The concentration of the NaOH used was 0.25M) Record your answer on your data form.
Determine the Molarity (concentration) of HCl using the data you collected and the titration formula. (The concentration of the NaOH used was 0.25M) Record your answer on your data form.
Questions
b. Based on the molarity of the acid calculated above, what is the pH of the lake water?
- State the purpose of this experiment.
- Describe the function of the phenolphthalein in this experiment. How would this experiment be different if you had forgotten to add the phenolphthalein
- State evidence from the lab that the endpoint was reached.
- It takes 75ml of a 2.5M HCl solution to neutralize 55ml of a base of unknown concentration. Calculate the concentration of an unknown basic solution.
- If the actual concentration of the HCl was 0.33M what is your percent error?
- Environmental studies usually involve an analysis of precipitation and its response to pollution. To quantify the degree of contamination in natural rain water or snow, titration is used. The process is quick and results are reliable. Since most titration processes do not require expensive or specialized equipment, the test can be performed often and in different areas with relatively little effort.
b. Based on the molarity of the acid calculated above, what is the pH of the lake water?