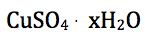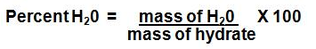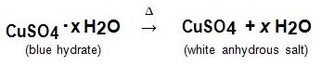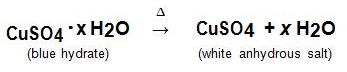Virtual Lab: Hydrates
Background:
Hydrates are ionic compounds (salts) that have a definite amount of water (water of hydration) as part of their structure. The water is chemically combined with the salt in a definite ratio. Ratios vary in different hydrates but are specific for any given hydrate.
The formula of a hydrate is represented in a special manner. The hydrate of copper sulfate in this experiment is listed below:
Hydrates are ionic compounds (salts) that have a definite amount of water (water of hydration) as part of their structure. The water is chemically combined with the salt in a definite ratio. Ratios vary in different hydrates but are specific for any given hydrate.
The formula of a hydrate is represented in a special manner. The hydrate of copper sulfate in this experiment is listed below:
In the formula, the unit formula for the salt appears first, and the water formula is last. The raised dot means that the water is loosely bonded to the salt. The coefficient x stands for the number of molecules of water bonded to one unit of salt. This special formula, like all other formulas, illustrates the law of definite composition.
When hydrates are heated, the "water of hydration" is released as vapor. The remaining solid is known as the anhydrous salt. The general reaction for heating a hydrate is:
When hydrates are heated, the "water of hydration" is released as vapor. The remaining solid is known as the anhydrous salt. The general reaction for heating a hydrate is:
Some anhydrous salts can absorb moisture from the air to become hydrated. A common example is silica gel, made from sodium silicate which is usually packaged with optical or electronic devices shipped by boat. The silica gel protects the devices from high humidity.

We can find the percent of water in a hydrate experimentally by accurately determining the mass of the hydrate and the mass of the anhydrous salt. The difference in mass is due to the water lost by the hydrate. The percentage of water in the original hydrate can easily be calculated:
In this experiment, as was mentioned, a hydrate of copper sulfate (blue) will be studied. The change from hydrate to anhydrous salt is accompanied by a change in color:
This investigation should aid you in your understanding of the formulas and composition of hydrates and the law of definite composition.
PURPOSE:
To determine the percentage of water in a hydrate.
PROCEDURE:
When copper (II) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water molecules and the blue solid is transformed to a white anhydrous (no water) crystal known as copper (II) sulfate.
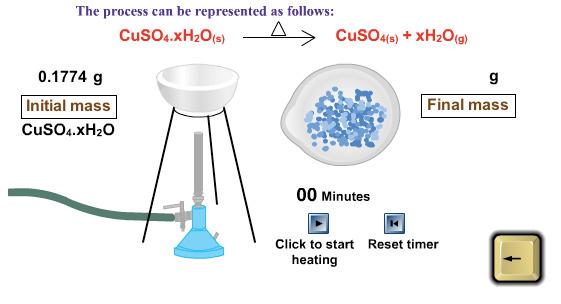
Use the computer simulation to determine the percentage of water in copper (II) sulfate hydrate.
The computer simulation can be found by clicking on the URL below:
introchem.chem.okstate.edu/DCICLA/Empirical.html
PURPOSE:
To determine the percentage of water in a hydrate.
PROCEDURE:
When copper (II) sulfate hydrate, a blue crystalline solid containing embedded water molecules (called a hydrate), is heated in air, it loses the water molecules and the blue solid is transformed to a white anhydrous (no water) crystal known as copper (II) sulfate.
Use the computer simulation to determine the percentage of water in copper (II) sulfate hydrate.
The computer simulation can be found by clicking on the URL below:
introchem.chem.okstate.edu/DCICLA/Empirical.html
DATA:
Initial Mass of Hydrate: _____________
Final Mass of Anhydrous Salt: _____________
CALCULATIONS:
1. Determine the mass of Water in the hydrate: ____________
2. Determine the percent of water in the hydrate: ______________
(Hint: Use percent compositon formula in Table T of reference table):
3. Determine the number of moles of H20 in the hydrate: ____________
(Hint: First determine GFM then use mole calculation in Table T of reference table)
4. Determine the number of moles of the anhydrous Salt: ____________
(Hint: First determine GFM then use mole calculation in Table T of reference table)
5. Using the reaction below determine the number of water molecules per mole of copper (II) sulfate hydrate:
CuSO4 . xH2O Determine "x", where x is a whole number.
Hint: step 1- Use the number of moles of water and copper sulfate calculated above
step 2- Divide by the smallest mole number
step 3- Round to the nearest whole number
****Special Thanks to the chemical education research group at Iowa State University for the amazing animation that you just used.