Virtual Lab: Precision and Significant Figures
Background:
Uncertainty in Measurement:
It is important to realize that any measurement will always contain some degree of uncertainty. The uncertainty of the measurement is determined by the scale of the measuring device. The smaller the unit you use to measure with, the more precise the measurement is.
Precision vs. Accuracy:
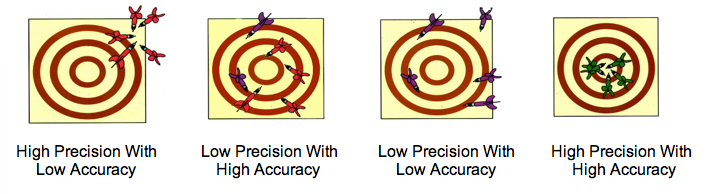
Precision- how close individual measurements agree with each other.
Accuracy- how close individual measurements agree with the true or accepted value.
Uncertainty in Measurement:
It is important to realize that any measurement will always contain some degree of uncertainty. The uncertainty of the measurement is determined by the scale of the measuring device. The smaller the unit you use to measure with, the more precise the measurement is.
Precision vs. Accuracy:
Precision- how close individual measurements agree with each other.
Accuracy- how close individual measurements agree with the true or accepted value.
Accurate number = small errors
Precise number = small uncertainty
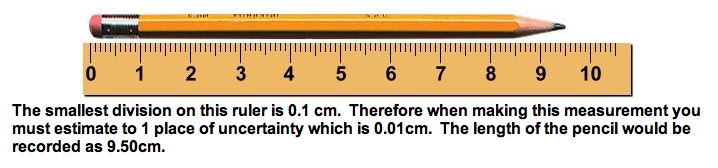
In General, the uncertainty of a measurement is determined by the precision of the measuring device. The smaller the unit you use to measure with, the more precise the measurement is. For example a 100mL graduated cylinder with 1mL graduation will have an uncertainty of +/- 0.1 mL. When making a measurement you must always estimate 1 place past the smallest divison.
Significant Figures
An alternative method of regarding uncertainty. In any measurement, the number of significant figures is critical. The number of significant figures is the number of digits believed to be correct by the person doing the measuring. It includes one estimated (uncertain) digit.
Rules for Working with Significant Figures:
1. Leading zeros are never significant.
Trapped zeros are always significant.
Trailing zeros are significant only if the decimal point is specified.
Hint: Change the number to scientific notation. It is easier to see.
2. Addition or Subtraction:
The last digit retained is set by the first doubtful digit.
3. Multiplication or Division:
The answer contains no more significant figures than the least accurately known number.
Pre Lab:
How Many Significant Figures Do These Numbers Contain?
Precise number = small uncertainty
In General, the uncertainty of a measurement is determined by the precision of the measuring device. The smaller the unit you use to measure with, the more precise the measurement is. For example a 100mL graduated cylinder with 1mL graduation will have an uncertainty of +/- 0.1 mL. When making a measurement you must always estimate 1 place past the smallest divison.
Significant Figures
An alternative method of regarding uncertainty. In any measurement, the number of significant figures is critical. The number of significant figures is the number of digits believed to be correct by the person doing the measuring. It includes one estimated (uncertain) digit.
Rules for Working with Significant Figures:
1. Leading zeros are never significant.
Trapped zeros are always significant.
Trailing zeros are significant only if the decimal point is specified.
Hint: Change the number to scientific notation. It is easier to see.
2. Addition or Subtraction:
The last digit retained is set by the first doubtful digit.
3. Multiplication or Division:
The answer contains no more significant figures than the least accurately known number.
Pre Lab:
How Many Significant Figures Do These Numbers Contain?
Click the link to check your answers: Answers
Objective:
The aim of this lab is to practice recording measurements with the correct precision.
Procedure:
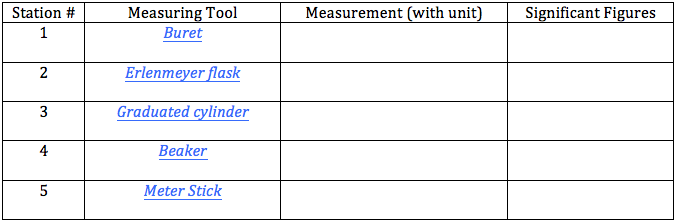
Several stations have been set up in this virtual lab. For each station, click on the measuring tool link, read the directions, and read the measurements according to the directions. Record this measurements with the correct units in the slot in your data table that corresponds to the station number. Remember to estimate to 1 place of uncertainty for each measurement. Then determine the number of significant figures for each measurement.
Lab Quiz:
1. Round off the following measurement to three significant figures.
1.296 g
2. How many significant figures are there in the following measurement?
2020 g
3. Round off the following measurement to three significant figures.
5.658 grams
1. Round off the following measurement to three significant figures.
1.296 g
2. How many significant figures are there in the following measurement?
2020 g
3. Round off the following measurement to three significant figures.
5.658 grams