Virtual Inquiry Lab: Atomic Structure and Electromagnetic Radiation
Background:
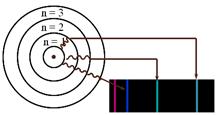
Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. As a result, each orbit has a fixed energy called an energy level. These energy levels (orbits) are like the rungs of a ladder (See Figure A). The electrons cannot be found in between these energy levels, just like a person cannot stand in between the rungs. Electrons must gain energy (become excited) to move up energy levels. Electrons can become excited by heat, light, electricity etc. These high energy electrons are unstable and eventually fall back down to their lower energy levels (ground states) releasing the energy that they had gained when they were initially excited. This energy is released in the form of light and it is what Bohr observed.
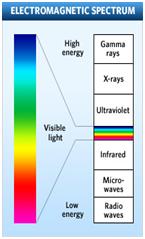
White light will produce a continuous spectrum when viewed using a prism or spectroscope (see Figure B). Different colors of light have different energies. When electrons of an atom are excited they will release different colors of light as they fall back to ground state (this is called emission line spectra). The colors of light correspond to the amount of energy released (See Figure C). Using a spectroscope you can view the emission line spectra. Different elements produce different spectra that are unique enough to be considered a “fingerprint” of the element
Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. As a result, each orbit has a fixed energy called an energy level. These energy levels (orbits) are like the rungs of a ladder (See Figure A). The electrons cannot be found in between these energy levels, just like a person cannot stand in between the rungs. Electrons must gain energy (become excited) to move up energy levels. Electrons can become excited by heat, light, electricity etc. These high energy electrons are unstable and eventually fall back down to their lower energy levels (ground states) releasing the energy that they had gained when they were initially excited. This energy is released in the form of light and it is what Bohr observed.
White light will produce a continuous spectrum when viewed using a prism or spectroscope (see Figure B). Different colors of light have different energies. When electrons of an atom are excited they will release different colors of light as they fall back to ground state (this is called emission line spectra). The colors of light correspond to the amount of energy released (See Figure C). Using a spectroscope you can view the emission line spectra. Different elements produce different spectra that are unique enough to be considered a “fingerprint” of the element
Objective:
Using a flame test and a spectroscope, determine the emission line spectrum of various known ions. Then determine the identity of 2 unknown ions using a flame test and the emission line spectra from the known ions.
Your Task:
Research Question:
What are the identities of the 2 unknown ion solutions?
Using a flame test and a spectroscope, determine the emission line spectrum of various known ions. Then determine the identity of 2 unknown ions using a flame test and the emission line spectra from the known ions.
Your Task:
- In your lab notebook decide what data you will need to collect in order to answer the research question. Develop your procedures and decide how you will collect your data.
- Perform the virtual experiment and analyze your results.
- Develop a scientific argument (claim, evidence, reasoning) that answers the research question. Write your argument in your lab notebook.
Research Question:
What are the identities of the 2 unknown ion solutions?
Emission Spectroscopy (known)
Click on the list of ions below to view their emission line spectra. Record your observations in your lab notebook.
Flame Test (known)
Click on the List of ions below to see the results of the flame test. Record Your Observations In Your Lab Notebook.
Emission Spectroscopy (unknown)
Click On The List Of Unknowns Below To View Their Emission Line Spectra. Record Your Observations In Your Lab Notebook.
Click On The List Of Unknowns Below To View Their Emission Line Spectra. Record Your Observations In Your Lab Notebook.
Flame Test (Unknown)
Click on the List of Unknowns below to see the results of the flame test. Record Your Observations In Your Lab Notebook.
***Adapted from Argument Driven Inquiry in Chemistry