Virtual Lab: Electrochemical Cells
Background:
Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be “oxidized” and the species that gain electrons is said to be “reduced”. A species cannot gain electrons unless another has lost electrons and vice versa. Oxidation and reduction go hand in hand. There are two major types of electrochemical cells: voltaic (also galled galvanic) and electrolytic. Voltaic cells produce electricity by harnessing the energy present in the flowing electrons. These reactions are spontaneous. Electrolytic cells use electrical energy to drive a redox reaction that normally would not occur because it is nonspontaneous.
Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be “oxidized” and the species that gain electrons is said to be “reduced”. A species cannot gain electrons unless another has lost electrons and vice versa. Oxidation and reduction go hand in hand. There are two major types of electrochemical cells: voltaic (also galled galvanic) and electrolytic. Voltaic cells produce electricity by harnessing the energy present in the flowing electrons. These reactions are spontaneous. Electrolytic cells use electrical energy to drive a redox reaction that normally would not occur because it is nonspontaneous.
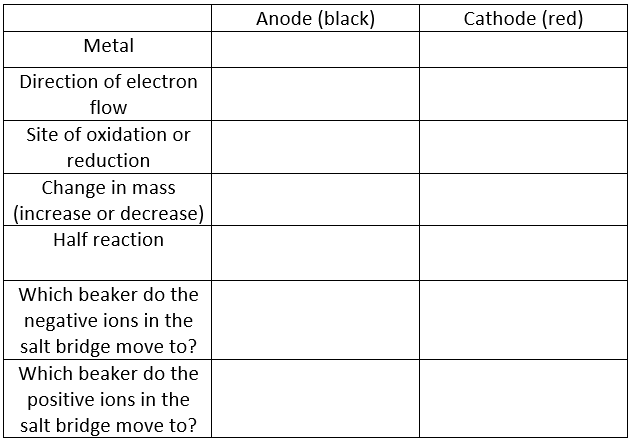
Part 1: Voltaic Cells
- Go to teachchemistry.org/classroom-resources/voltaic-cells
- Click START
- For the experiment setup, in the left beaker select "copper" and for the right beaker select "zinc"
- Click ON
- Click on "see molecular scale" for each beaker and the salt bridge to see what is occuring at the molecular level and fill in the table below.
Analysis Questions: Part 1
- What is the function of the salt bridge?
- What do electrons flow through?
- In terms of atoms, ions and electrons, explain why the mass decreased at one electrode and increased at the other.
- If you made a new voltaic cell with Zn and Ag electrodes, what metal would be the anode and which would be the cathode?
- In this new cell, what electrode would be oxidized and which will be reduced?
- In this new cell, what direction would electrons flow?
- Write the half reaction that occurs at the anode and cathode.
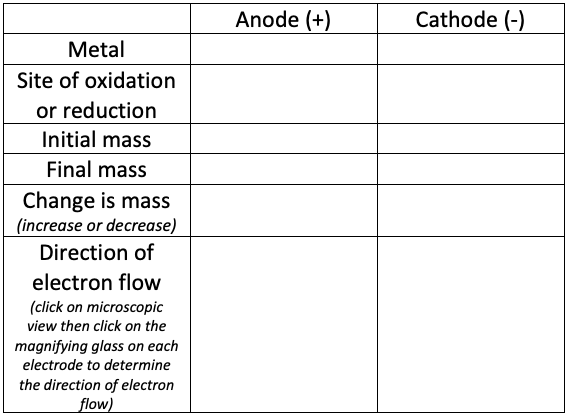
Part 2: Electrolytic Cell (Electroplating)
- Go to media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/html5/Electro/Electro.php
- Click on "experiment" then click "run demonstration."
- Follow the instructions in the demonstration and fill in the data table below as you go through it.
Analysis Questions Part 2:
1. Is electroplating a spontaneous reaction, or does it require energy? (Look at the voltage)
2. Write the half reaction that occurs at the anode.
3. Write the half reaction that occurs at the cathode.
4. Explain the differences and similarities between the voltaic cell in Part 1 and the electrolytic cell in Part 2.
1. Is electroplating a spontaneous reaction, or does it require energy? (Look at the voltage)
2. Write the half reaction that occurs at the anode.
3. Write the half reaction that occurs at the cathode.
4. Explain the differences and similarities between the voltaic cell in Part 1 and the electrolytic cell in Part 2.
Looking for a more detailed explanation of how the voltaic cell works? Click here