Acid Base Inquiry Lab Using Red Cabbage
Introduction:
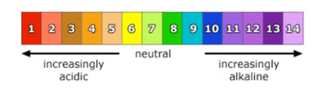
Liquids all around us have either acidic or basic (alkaline) properties. For example, acids taste sour; while, bases taste bitter and feel slippery. However, both strong acids and strong bases can be very dangerous and burn your skin, so it is important to be very careful when using such chemicals. In order to measure how acidic or basic a liquid is, one must use the pH scale as illustrated below:
Liquids all around us have either acidic or basic (alkaline) properties. For example, acids taste sour; while, bases taste bitter and feel slippery. However, both strong acids and strong bases can be very dangerous and burn your skin, so it is important to be very careful when using such chemicals. In order to measure how acidic or basic a liquid is, one must use the pH scale as illustrated below:
The strength of the pH scale is determined by the concentration of hydrogen ions (H+) where a high concentration of H+ ions indicate a low pH and a low concentration of H+ ions indicate a high pH. The pH scale ranges from 1 to 14 where 1 to 6 is classified as acidic, 7 neutral (neither a base nor an acid) and 8 to 14 is classified as basic.
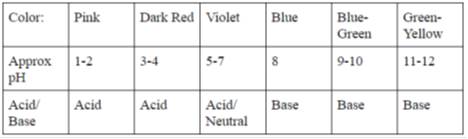
In this lab, you will use the juice from red cabbage as a pH indicator to test common household liquids and determine their pH levels. You will mix cabbage juice with different household liquids and see a color change produced by a pigment called flavin (an anthocyanin) in red cabbage. Through this color change, you will be able to successfully identify the approximate pH of common household liquids using the table below:
In this lab, you will use the juice from red cabbage as a pH indicator to test common household liquids and determine their pH levels. You will mix cabbage juice with different household liquids and see a color change produced by a pigment called flavin (an anthocyanin) in red cabbage. Through this color change, you will be able to successfully identify the approximate pH of common household liquids using the table below:
Table 1:
Pre-Lab Questions
Look at each of the liquids being tested. Predict whether each of the substances is acidic, neutral or basic. Circle one. (Think about the properties of acids and bases.)
Hand Sanitizer Acidic Neutral Basic
Lemon Juice Acidic Neutral Basic
Apple Juice Acidic Neutral Basic
White Vinegar Acidic Neutral Basic
Baking Soda Acidic Neutral Basic
Shampoo Acidic Neutral Basic
Conditioner Acidic Neutral Basic
Look at each of the liquids being tested. Predict whether each of the substances is acidic, neutral or basic. Circle one. (Think about the properties of acids and bases.)
Hand Sanitizer Acidic Neutral Basic
Lemon Juice Acidic Neutral Basic
Apple Juice Acidic Neutral Basic
White Vinegar Acidic Neutral Basic
Baking Soda Acidic Neutral Basic
Shampoo Acidic Neutral Basic
Conditioner Acidic Neutral Basic
Procedure: For each substance, click on the liquid to observe the color change of the red cabbage juice. Using table 1 above, determine the approximate pH of the substance.
- Click to see the effect of Hand Sanitizer on the red cabbage juice
- Click to see the effect of lemon Juice on the red cabbage juice
- Click to see the effect of Apple Juice on the red cabbage juice
- Click to see the effect of White Vinegar on the red cabbage juice
- Click to see the effect of Baking Soda on the red cabbage juice
- Click to see the effect of Shampoo on the red cabbage juice
- Click to see the effect of Conditioner on the red cabbage juice
Conclusion Questions: (Use the internet and the lab results to answer the following questions)
- In terms of acids and bases, what does neutralization mean?
- How does a pH of 3 differ from a pH of 4 in terms of H+ concentration? Which is stronger?
- Neutralization: Whenever you mix an acid with a base, they neutralize each other. If this is the case, why is Alka-Seltzer used to treat stomach aches? (Note: excess stomach acids cause stomach aches)
- Acid Rain: What is acid rain and how is it a problem to oceans, rivers, lakes, ponds etc.?
References:
Acids and Bases: http://www.chem4kids.com/files/react_acidbase.html
pH image: http://www.pullouttheplug.co.uk/.../ph-scale.gif
Red Cabbage Juice Lab: http://www.curriki.org/xwiki/bin/view/Coll_MickiHR/AcidsandBases
Lab edit: http://web.stanford.edu/~ajspakow/downloads/outreach/ph-student-9-30-09.pdf
Acids and Bases: http://www.chem4kids.com/files/react_acidbase.html
pH image: http://www.pullouttheplug.co.uk/.../ph-scale.gif
Red Cabbage Juice Lab: http://www.curriki.org/xwiki/bin/view/Coll_MickiHR/AcidsandBases
Lab edit: http://web.stanford.edu/~ajspakow/downloads/outreach/ph-student-9-30-09.pdf