Virtual Lab: Spectroscopy
| virtual_lab_spectroscopy.pdf | |
| File Size: | 365 kb |
| File Type: | |
Background:
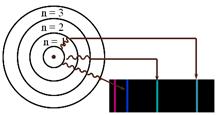
Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. As a result, each orbit has a fixed energy called an energy level. These energy levels (orbits) are like the rungs of a ladder (See Figure A). The electrons cannot be found in between these energy levels, just like a person cannot stand in between the rungs. Electrons must gain energy (become excited) to move up energy levels. Electrons can become excited by heat, light, electricity etc. These high energy electrons are unstable and eventually fall back down to their lower energy levels (ground states) releasing the energy that they had gained when they were initially excited. This energy is released in the form of light and it is what Bohr observed.
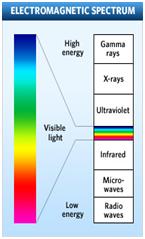
White light will produce a continuous spectrum when viewed using a prism or spectroscope (see Figure B). Different colors of light have different energies. When electrons of an atom are excited they will release different colors of light as they fall back to ground state (this is called emission line spectra). The colors of light correspond to the amount of energy released (See Figure C). Using a spectroscope you can view the emission line spectra. Different elements produce different spectra that are unique enough to be considered a “fingerprint” of the element
Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. As a result, each orbit has a fixed energy called an energy level. These energy levels (orbits) are like the rungs of a ladder (See Figure A). The electrons cannot be found in between these energy levels, just like a person cannot stand in between the rungs. Electrons must gain energy (become excited) to move up energy levels. Electrons can become excited by heat, light, electricity etc. These high energy electrons are unstable and eventually fall back down to their lower energy levels (ground states) releasing the energy that they had gained when they were initially excited. This energy is released in the form of light and it is what Bohr observed.
White light will produce a continuous spectrum when viewed using a prism or spectroscope (see Figure B). Different colors of light have different energies. When electrons of an atom are excited they will release different colors of light as they fall back to ground state (this is called emission line spectra). The colors of light correspond to the amount of energy released (See Figure C). Using a spectroscope you can view the emission line spectra. Different elements produce different spectra that are unique enough to be considered a “fingerprint” of the element
Objective:
In this virtual lab you will:
1.Observe the bright line spectra (emission spectra) for various elements.
2.Use a flame test to observe the color produced when metal ions are heated.
3.Identify unknown metals ions based on the results of the flame test.
Procedure:
Part I. (Bright Line Spectra)
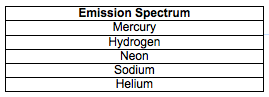
Write the name of each element located in Part I. into data table 1 located below. Then click on each element and observe the line spectra that are produced. In data table 1 draw lines under each letter representing the colors that you observed. (R= red, O= orange, Y= yellow, G= green, B= blue & V= violet…..Indigo was left out because it is difficult to distinguish in this activity).
Part II. (Flame Test)
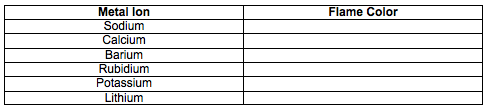
Write the name of each metal ion located in Part II. Into data table 2 located below. Then click on each element to observe the color produced from the flame test. Record your results into table 2 located below. Using these results click on the two unknowns and record their flame colors. Identify the name of each unknown metal ion based upon your flame test results.
In this virtual lab you will:
1.Observe the bright line spectra (emission spectra) for various elements.
2.Use a flame test to observe the color produced when metal ions are heated.
3.Identify unknown metals ions based on the results of the flame test.
Procedure:
Part I. (Bright Line Spectra)
Write the name of each element located in Part I. into data table 1 located below. Then click on each element and observe the line spectra that are produced. In data table 1 draw lines under each letter representing the colors that you observed. (R= red, O= orange, Y= yellow, G= green, B= blue & V= violet…..Indigo was left out because it is difficult to distinguish in this activity).
Part II. (Flame Test)
Write the name of each metal ion located in Part II. Into data table 2 located below. Then click on each element to observe the color produced from the flame test. Record your results into table 2 located below. Using these results click on the two unknowns and record their flame colors. Identify the name of each unknown metal ion based upon your flame test results.
Part I. Emission Spectroscopy
Click on the elements below to view the emission line spectra. Record your observations on your lab sheet.
Part II. Flame Test
Click on the metal ions below to see the results of the flame test. Record your observations on your lab sheet. Then click on each unknown and record the results of each flame test. Then identify the unknown by matching the flame color to one of the known metal ions.
Conclusion: (answer on your lab sheet)
1. What evidence is there that electrons move around in definite pathways around the nucleus?
2. How is emission spectra produced?
3. How might emission spectra be used in studying stars?
4. Draw a Bohr diagram for Hydrogen and Neon. (If you need help on drawing Bohr diagrams click on this link below)
1. What evidence is there that electrons move around in definite pathways around the nucleus?
2. How is emission spectra produced?
3. How might emission spectra be used in studying stars?
4. Draw a Bohr diagram for Hydrogen and Neon. (If you need help on drawing Bohr diagrams click on this link below)
5. Why do larger gases such as Neon produce more color bands (line spectra) than smaller gases like Hydrogen? ****(Hint- think about how many energy levels are in each element)
6. Explain how colors in the flame test are produced.
7. How are the electrons “excited” in Part 2 of the experiment?? What does it mean when the electrons are “excited”?
8. Explain why we did not see distinct lines (like the emission spectrum in Part I.) when the metal salts were burned.
9. Colorful light emissions are applicable to everyday life. Where else have you observed colorful light emissions? Are these light emission applications related? Explain.
6. Explain how colors in the flame test are produced.
7. How are the electrons “excited” in Part 2 of the experiment?? What does it mean when the electrons are “excited”?
8. Explain why we did not see distinct lines (like the emission spectrum in Part I.) when the metal salts were burned.
9. Colorful light emissions are applicable to everyday life. Where else have you observed colorful light emissions? Are these light emission applications related? Explain.