Virtual Lab: Electrochemical Cells
Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be “oxidized” and the species that gain electrons is said to be “reduced”. A species cannot gain electrons unless another has lost electrons and vice versa. Oxidation and reduction go hand in hand. There are two major types of electrochemical cells: voltaic (also galled galvanic) and electrolytic. Voltaic cells produce electricity by harnessing the energy present in the flowing electrons. These reactions are spontaneous. Electrolytic cells use electrical energy to drive a redox reaction that normally would not occur because it is nonspontaneous.
Part I: Standard Cell Potentials (Voltaic Cells)
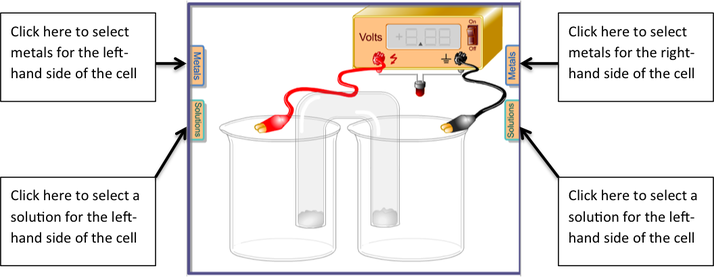
- Go to https://pages.uoregon.edu/tgreenbo/voltaicCellEMF.html Make the following voltaic cells: #1: Cu2+/Cu | | Ag+/Ag #2: Zn/Zn2+ | | Ag+/Ag #3: Zn/Zn2+ | | Cu2+/Cu
- For each of the above, place the metal in a solution of its own ions. Make sure the cells are set up so that the cell potential is a positive value, indicating that the voltaic cell is set up correctly and the redox reaction is spontaneous. (Hint: In this simulation, the anode is black and the cathode is red.)
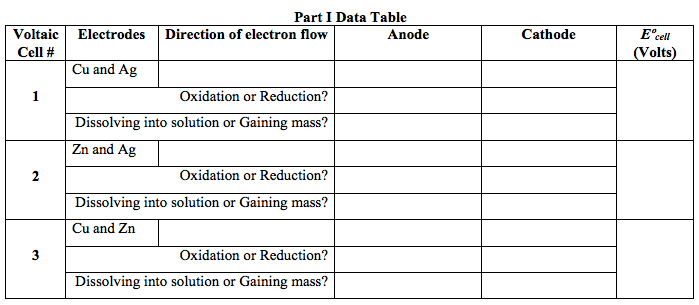
- For each of the three voltaic cells, record the direction of electron flow, determine which electrode is the anode and which is the cathode, and record the cell voltage in the table on the next page.
- For each electrode, determine whether oxidation or reduction is taking place. Record this in the table.
- For each electrode, determine whether the electrode is dissolving away (becoming an ion and going in to solution) OR gaining mass (ions in solution are becoming neutral atoms that are deposited on the electrode). Record this in the table.
- You must click the “Off” switch to reset for the next voltaic cell.
Analysis Questions: Part I (show setups for any calculations)
1. What is another name for a voltaic cell?
2. For the first cell, Cu-Ag:
a. Write the oxidation AND reduction half-reactions. Label each as “oxidation” or “reduction”.
b. Write the balanced, net ionic equation for the reaction.
3. For the second cell, Zn-Ag:
a. Write the oxidation AND reduction half-reactions. Label each as “oxidation” or “reduction”.
b. Write the balanced, net ionic equation for the reaction.
4. For the third cell, Zn-Cu:
a. Write the oxidation AND reduction half-reactions. Label each as “oxidation” or “reduction”.
b. Write the balanced, net ionic equation for the reaction.
1. What is another name for a voltaic cell?
2. For the first cell, Cu-Ag:
a. Write the oxidation AND reduction half-reactions. Label each as “oxidation” or “reduction”.
b. Write the balanced, net ionic equation for the reaction.
3. For the second cell, Zn-Ag:
a. Write the oxidation AND reduction half-reactions. Label each as “oxidation” or “reduction”.
b. Write the balanced, net ionic equation for the reaction.
4. For the third cell, Zn-Cu:
a. Write the oxidation AND reduction half-reactions. Label each as “oxidation” or “reduction”.
b. Write the balanced, net ionic equation for the reaction.
Part II: Electroplating (Electrolytic Cells)
- Go to http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/electroChem/electrolysis10.html
- Click run experiment. pages.uoregon.edu/tgreenbo/electrolysis10.html
- Construct a copper electroplating cell by placing a copper anode and iron cathode in a solution of Cu2+ ions. (anode is red and cathode is black)
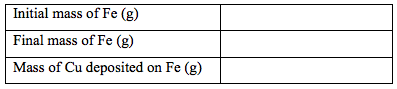
- Record the initial mass of the iron cathode in the data table.
- Run the simulation at a current of 2.00 amperes at 2.00 V for 5:00 minutes. Record the final mass of the iron cathode. Record in the data table and calculate the mass of copper deposited on the iron.
Analysis Questions: Part III (show setups for any calculations)
1. Is electroplating a spontaneous reaction, or does it require energy? (Look at the voltage)
2. What attracts the Cu onto the Fe electrode?
3. State the direction of electron flow through the circuit.
4. Calculate the moles of copper formed.
5. Write the Cu half reaction that takes place on the Fe electrode as Cu is deposited.
6. How many moles of electrons are transferred when one mole of Cu is formed?
7. Calculate the moles of electrons that ran through this circuit in order for the Cu to form. (Multiply the moles of Cu by the moles of electrons traveling).
1. Is electroplating a spontaneous reaction, or does it require energy? (Look at the voltage)
2. What attracts the Cu onto the Fe electrode?
3. State the direction of electron flow through the circuit.
4. Calculate the moles of copper formed.
5. Write the Cu half reaction that takes place on the Fe electrode as Cu is deposited.
6. How many moles of electrons are transferred when one mole of Cu is formed?
7. Calculate the moles of electrons that ran through this circuit in order for the Cu to form. (Multiply the moles of Cu by the moles of electrons traveling).